Background
The combination of the anti-CD19 monoclonal antibody tafasitamab and the immune modulatory drug lenalidomide (LEN) has been commercially available in the US for adult patients with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL) not otherwise specified, including DLBCL arising from low-grade lymphoma, and who are ineligible for autologous stem cell transplant (ASCT) since August 05, 2020.
It was granted accelerated FDA approval and EU conditional marketing authorization after the primary analysis of Phase II L-MIND study (NCT02399085) demonstrated safety and efficacy in this setting. To complement clinical data, it is important to assess real-world safety and effectiveness given the heterogeneity of routine clinical care. Furthermore, focusing on patients from racial and ethnic minorities, who may face challenges in receiving optimal access to care, may help to identify and address inequities. realMIND is an observational study of the real-world safety and effectiveness of tafasitamab + LEN for the treatment of R/R DLBCL among patients in the US by race and ethnicity.
Using prospective and, where necessary, retrospective data collection, this non-interventional study will also provide a deeper understanding of treatment patterns of US patients with R/R DLBCL treated with tafasitamab, and its use in a real-world setting in terms of treatment duration, dose modifications, and post-tafasitamab + LEN combination partners and monotherapy use.
Methods
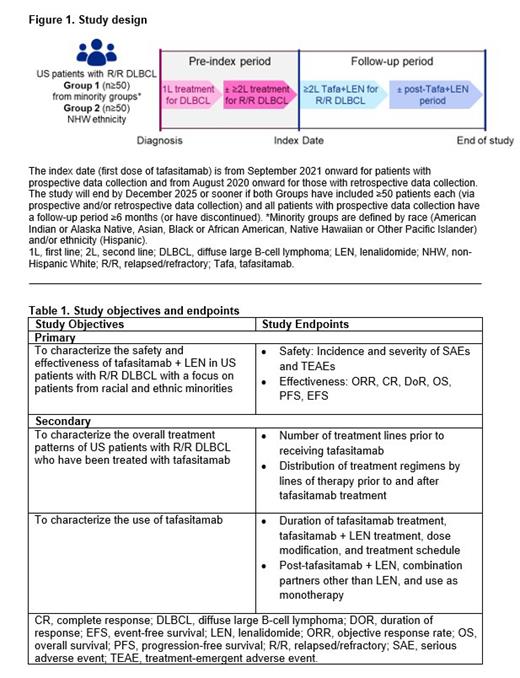
realMIND (NCT04981795) is a multicenter, observational, prospective and retrospective Phase IV trial-in-progress study targeting ≥100 US patients with R/R DLBCL treated with tafasitamab + LEN. To ensure diversity, Group 1 will enroll patients from racial and ethnic minorities (n≥50), while Group 2 will comprise non-Hispanic White (NHW) patients (n≥50) (Figure 1). Eligible patients are ≥18 years old
with histologically confirmed R/R DLBCL, ≥1 prior line of treatment, and have initiated or will be initiating tafasitamab treatment (except in the context of an interventional study). Prospective data collection began at study sites in September 2021, and retrospective data (from study sites or vendor databases) may include patients with a tafasitamab start date (index date) from August 05, 2020, onwards.
The primary endpoints include safety and effectiveness outcomes (Table 1). Serious adverse events (SAEs) and treatment-emergent adverse events (TEAEs) will be reported. Effectiveness will be assessed in terms of tumor response rates and time-to-event endpoints, while secondary endpoints include treatment line and dose information (Table 1). Patient data for this observational study will be collected by prospective
follow-up of patients included at study sites, or by retrospective collection of data from patient records, at study sites or from vendor databases. Prospective and retrospective data will be analyzed separately, as well as pooled.
No formal sample size calculation was performed due to the descriptive nature of the study and no hypothesis testing is planned.
Study status
The in-progress non-interventional realMIND study is expected to end by approximately December 2025, or earlier, when: ≥50 patients treated with tafasitamab + LEN have been recruited into each of Group 1 and Group 2 with either prospective or retrospective data collection; and all prospectively-followed patients have ≥6 months follow-up after starting tafasitamab treatment, or have discontinued from the study for any reason, whichever occurs earlier. The results are anticipated to describe the real-world safety and effectiveness of tafasitamab for R/R DLBCL in US patients, with a focus on underrepresented racial and ethnic minorities, to help optimize outcomes of treatment and identify opportunities for further studies.
Disclosures
Brem:ADC Therapeutics: Consultancy; Astra Zeneca: Consultancy, Speakers Bureau; AbbVie/GenMab: Speakers Bureau; SeaGen: Speakers Bureau; Caribou Bioscineces: Consultancy; Morphosys/Incyte: Consultancy, Speakers Bureau; BeiGene: Consultancy, Speakers Bureau. Burke:Epizyme: Consultancy; BeiGene: Consultancy, Speakers Bureau; Bristol Myers Squibb: Consultancy; Kymera: Consultancy; Roche/Genentech: Consultancy; Gilead Sciences: Consultancy; X4 Pharmaceuticals: Consultancy; Verastem: Consultancy; AstraZeneca: Consultancy; Adaptive Biotechnologies: Consultancy; Morphosys: Research Funding; AbbVie: Consultancy; Bayer HealthCare Pharmaceuticals: Consultancy; Kura Oncology: Consultancy; Seagen Inc.: Consultancy, Speakers Bureau; MorphoSys AG: Consultancy; Nurix: Consultancy. Vukcevic:MorphoSys AG: Current Employment. Kloepfer:MorphoSys AG: Current Employment. Saverno:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Abdulhaq:Oncopeptide, Morphosys, Genentech, Pfizer: Research Funding; Genentech: Speakers Bureau; Amgen, MorphoSys, Genentech, BMS, Novartis, Pfizer, AbbVie: Consultancy. Evens:ORIEN, Leukemia & Lymphoma Society.: Other: grant/research support, Research Funding; Novartis, AbbVie, Pharmacyclics, Seattle Genetics, Hutchmed, Incyte, Daiichi Sankyo, Epizyme; Curio, Cota, Patient Power, Curio Science, OncLive, Research to Practice: Consultancy. Porcu:Kymera: Membership on an entity's Board of Directors or advisory committees; Kyowa: Consultancy; BioGene: Membership on an entity's Board of Directors or advisory committees; Dren-Bio, ADCT, Lilly-Loxo, Viracta, Innate Pharma: Membership on an entity's Board of Directors or advisory committees; Ono: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa, Daiichi, Viracta, Dren Bio, Innate Pharma: Consultancy; Kyowa, Daiichi, Viracta, Dren Bio, Innate Pharma, Ono: Honoraria; Teva: Research Funding; Innate Pharma: Research Funding. Shadman:Mustang Bio, BMS, Pharmacyclics, Genentech, AbbVie, TG Therapeutics, BeiGene, AstraZeneca, Genmab, MorphoSys/Incyte, Vincerx: Research Funding; AbbVie, Genentech, AstraZeneca, Pharmacyclics, BeiGene, BMS, MorphoSys/Incyte, Kite, Eli Lilly, Genmab, Mustang Bio, Regeneron, ADC therapeutics, Fate Therapeutics, Janssen and MEI Pharma: Consultancy. Flowers:Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Denovo Biopharma: Consultancy; TG Therapeutics: Research Funding; Allogene: Research Funding; Amgen: Research Funding; Iovance: Research Funding; Burroghs Wellcome Fund: Research Funding; Xencor: Research Funding; Bayer: Consultancy, Research Funding; Pharmacyclics Jansen: Consultancy; SeaGen: Consultancy; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Jannsen Pharmaceuticals: Research Funding; Kite: Research Funding; Acerta: Research Funding; Guardant: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; CPRIT Scholar in Cancer Research: Research Funding; V Foundation: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Cellectis: Research Funding; Genentech Roche: Consultancy, Research Funding; National Cancer Institute: Research Funding; Abbvie: Consultancy, Research Funding; Beigene: Consultancy; Ziopharm: Research Funding; Nektar: Research Funding; Celgene: Consultancy, Research Funding; Takeda: Research Funding; Pharmacyclics: Research Funding; Sanofi: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Karyopharm: Consultancy; Spectrum: Consultancy; 4D: Research Funding; Adaptimmune: Research Funding; Gilead: Consultancy, Research Funding; Genmab: Consultancy; Morphosys: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal